Loading...
Meet the ULSPIRA™ Solution
A complete nitric oxide therapy offering.
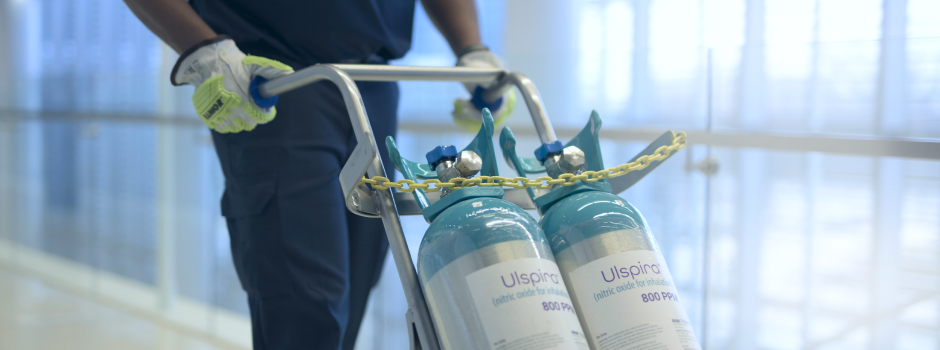
Time is of the essence, especially when caring for your littlest and most vulnerable patients in the NICU. When you have a patient experiencing hypoxic respiratory failure, you need to act fast. You can’t afford to scramble to prepare your equipment, or replace your cylinders, or find the right consumables for the prescribed therapy. You need to ensure that everything is at your fingertips and ready to go quickly.
Removing these barriers to patient care allows you to focus on what is most important — your patients.
Introducing the ULSPIRA solution — a complete nitric oxide (NO) therapy offering designed to help you deliver a higher level of patient care.
The ULSPIRA solution offers customizable support services, an easy‑to‑use, feature‑rich NO delivery system designed for simplicity and safety, and our FDA-approved nitric oxide gas.
Click to learn more about the ULSPIRA solution
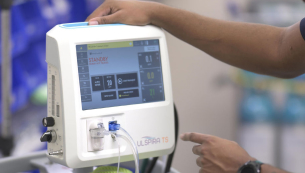
ULSPIRA TS™
The ULSPIRA TS Nitric Oxide Therapy System was designed for ease of use with patient safety in mind, providing simplified treatment management to bring inhaled NO therapy to the next level. Our therapy system is supported by our team of clinical applications specialists who are trained RTs with years of nitric oxide therapy experience.
Save time and start therapy fast
- Short pre-use check with onscreen instructions
- Automatic cylinder purge and switch
- Single-use patient consumable kits
Simplify treatment management
- Intuitive touch screen interface
- Flow sensing modes, including Auto and Jet Sense, and Constant Rate mode
- Compatible with a wide range of flows
Focus on patient safety
- Emergency Dosing modes
- Precise dosing in 0.1 ppm increments to 10 ppm
- Integrated pneumatic backup system
Clinical support for your team
24/7/365 access to Clinical Application Specialists (RRT) via dedicated hotline: 833-ULSPIRA (833-857-7472)
- Clinical education on device and applications
- Complete user training and support
- On-going clinical support and resources

ULSPIRA Care+™
Put the + in your care. ULSPIRA Care+ is a human solution to remove even more of your burden of care when delivering nitric oxide therapy to patients.
Always there. 24/7/365 access to Clinical Application Specialists (RRT).
Customized, on–site support. Utilizing Airgas Therapeutics human resources to perform routine tasks, easing the burden of care for Respiratory Therapists and allowing hospital staff to focus on patient care.
Patient-centric, value-based business approach: Available per treatment business model, made possible by novel patient treatment insights.
ULSPIRA Care+ is our human solution utilizing Airgas resources to perform routine tasks, easing the burden of care for Respiratory Therapists.

Focus on Safety
Cylinder management and transport

Optimize RT Time
High calibrations performed for you at your request

Minimize Emergencies
Inventory and supply management
Always Ready
Device preventive maintenance management
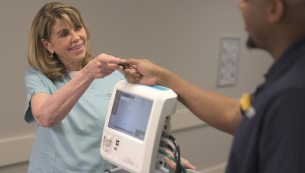
Patient-centric Approach
Per patient treatment data collection and sharing
ULSPIRA™
ULSPIRA (nitric oxide) gas for inhalation is a vasodilator drug indicated to improve oxygenation and reduce the need for extracorporeal membrane oxygenation in term and near-term (>34 weeks gestation) neonates with hypoxic respiratory failure associated with clinical or echocardiographic evidence of pulmonary hypertension in conjunction with ventilatory support and other appropriate agents. ULSPIRA is available in a 800 ppm concentration.

LEARN MORE ABOUT ULSPIRA
Fill in the form below to contact an expert for your ULSPIRA needs.
Thank You!
An Airgas Therapeutics representative will contact you soon!
ULSPIRA Indication and Important Safety Information
ULSPIRA (nitric oxide) gas, for inhalation, is a vasodilator indicated to improve oxygenation and reduce the need for extracorporeal membrane oxygenation in term and near-term (>34 weeks gestation) neonates with hypoxic respiratory failure associated with clinical or echocardiographic evidence of pulmonary hypertension in conjunction with ventilatory support and other appropriate agents.
Contraindications
ULSPIRA is contraindicated in neonates dependent on right-to-left shunting of blood.
Warnings and Precautions
- Rebound: Abrupt discontinuation of ULSPIRA may lead to worsening oxygenation and increasing pulmonary artery pressure.
- Methemoglogin: Methemoglobin increases with the dose of ULSPIRA and is dose dependent. Following discontinuation or reduction of nitric oxide, methemoglobin levels can take up to 8 hours or more to return to baseline. If methemoglobin levels do not resolve with decrease in dose or discontinuation of ULSPIRA, additional therapy may be warranted to treat methemoglobinemia.
- Elevated Levels: Monitor for PaO2, inspired nitrogen dioxide (NO2), and methemoglobin during ULSPIRA administration.
- Heart Failure: In patients with pre-existing left ventricular dysfunction, ULSPIRA may increase pulmonary capillary wedge pressure, leading to pulmonary edema.
- Nitrogen dioxide may cause airway inflammation and damage to lung tissues.
Adverse Reactions
The most common adverse reaction of ULSPIRA is hypotension.
Drug Interactions
Nitric Oxide donor compounds may increase the risk of developing methemoglobinemia.
Use With Delivery System
ULSPIRA must be administered using a calibrated FDA-cleared Nitric Oxide Delivery System operated by trained personnel. Only validated ventilator systems should be used in conjunction with ULSPIRA.
Adverse Events
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please see full ULSPIRA prescribing information for additional ULSPIRA safety and risk information.
ULSPIRA TS Safety Statement
Intended Use
The ULSPIRA TS Nitric Oxide Therapy System is intended for use by healthcare professionals for the delivery of nitric oxide (NO) and the monitoring of inspired NO, NO2 and O2 concentrations for a patient undergoing inhaled Nitric Oxide (iNO) therapy.
ULSPIRA TS must only be used in accordance with the indications, contraindications, warnings and precautions described in the nitric oxide drug packaging inserts and labeling, and is indicated for use in term and near-term (>34 weeks gestation) neonates with hypoxic respiratory failure associated with clinical or echocardiographic evidence of pulmonary hypertension. The primary targeted clinical setting is the Neonatal Intensive Care Unit (NICU) and secondary targeted clinical setting is the transport of neonates. Refer to this material prior to use.
In the case of an abrupt stop of inhaled NO gas delivery, withdrawal (rebound pulmonary hypertension) may occur. Possible side-effects of the inhaled NO gas therapy are further described in the nitric oxide drug packaging inserts and labeling.
ULSPIRA TS can be used with respiratory devices. Make sure that ULSPIRA TS is validated with the respiratory device you use. Validated respiratory devices are listed in the User's Manual.
Device Warnings:
- Persons using ULSPIRA TS must be trained and experienced in its use to ensure effective administration of nitric oxide and to avoid injury to the patient or to others resulting from inhalation of excess NO, NO2 or other reaction products.
- Always perform a Pre-Use Check before connecting ULSPIRA TS to the patient. Only use the system if the Pre-Use Check is successfully passed.
- If an unintended treatment interruption occurs, use the backup system.
- ULSPIRA TS must not be used in an MRI environment.
Rx Only
ULSPIRA Indication and Important Safety Information
ULSPIRA (nitric oxide) gas, for inhalation, is a vasodilator indicated to improve oxygenation and reduce the need for extracorporeal membrane oxygenation in term and near-term (>34 weeks gestation) neonates with hypoxic respiratory failure associated with clinical or echocardiographic evidence of pulmonary hypertension in conjunction with ventilatory support and other appropriate agents.
Contraindications
ULSPIRA is contraindicated in neonates dependent on right-to-left shunting of blood.
Warnings and Precautions
- Rebound: Abrupt discontinuation of ULSPIRA may lead to worsening oxygenation and increasing pulmonary artery pressure.
- Methemoglogin: Methemoglobin increases with the dose of ULSPIRA and is dose dependent. Following discontinuation or reduction of nitric oxide, methemoglobin levels can take up to 8 hours or more to return to baseline. If methemoglobin levels do not resolve with decrease in dose or discontinuation of ULSPIRA, additional therapy may be warranted to treat methemoglobinemia.
- Elevated Levels: Monitor for PaO2, inspired nitrogen dioxide (NO2), and methemoglobin during ULSPIRA administration.
- Heart Failure: In patients with pre-existing left ventricular dysfunction, ULSPIRA may increase pulmonary capillary wedge pressure, leading to pulmonary edema.
- Nitrogen dioxide may cause airway inflammation and damage to lung tissues.
Adverse Reactions
The most common adverse reaction of ULSPIRA is hypotension.
Drug Interactions
Nitric Oxide donor compounds may increase the risk of developing methemoglobinemia.
Use With Delivery System
ULSPIRA must be administered using a calibrated FDA-cleared Nitric Oxide Delivery System operated by trained personnel. Only validated ventilator systems should be used in conjunction with ULSPIRA.
Adverse Events
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please see full ULSPIRA prescribing information for additional ULSPIRA safety and risk information.